Target Clinical Description
(Original ideas for this phenotype was described in a 10 minute video here https://www.youtube.com/watch?v=8ksrXjT1faU )
- Anaphylaxis Anaphylaxis - StatPearls - NCBI Bookshelf 1
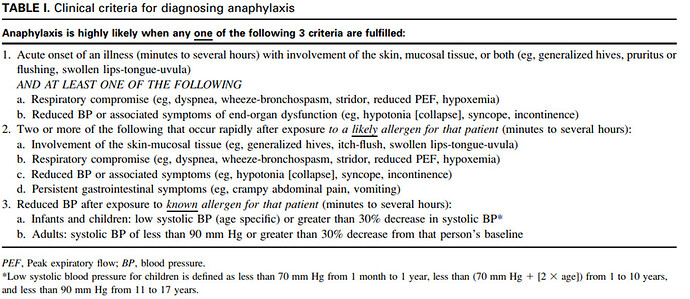
Summary: Acute potentially life-threatening multisystem syndrome due to mast cell mediator release most often via IgE. Presentation: Anaphylaxis is highly likely when sampson criteria is satisficed. Anaphylaxis is a clinical diagnosis, and labs are not used as part of diagnosis. Plan: Preserve airway and breathing, circulation, mentation. Evaluate for airway compromise. Epinephrine IM or IV depending on severity of presentation. Antihistamines as adjunct. Bronchodilators for bronchospasm. Prognosis: Complete recovery common when anaphylaxis recognized early in the clinical course, to fatal if treated too late or with inadequate intervention. Allergist consult recommended after recovery.
Sampson Criteria
Additional case definitions are discussed here Anaphylaxis: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data - ScienceDirect
MedDRA PTs: anaphylactic reaction, anaphylactoid reaction, anaphylactic shock, anaphylactoid shock
Phenotype Development: We made design choices in the cohort definition based on the following reasons.
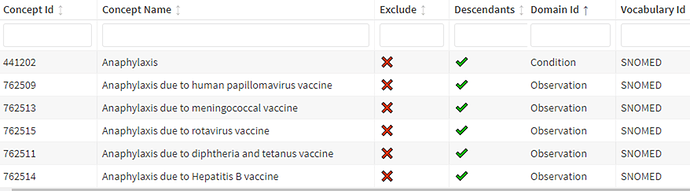
1. Concept Set expression creation: We chose the following concept id based on lexical matching. Only one concept id is from the condition domain, and others are from observation domain.
2. No visit restriction: we decided to not restrict persons based on visit.
3.Exit strategy: Because most persons with anaphylaxis are expected to not continuously suffer from anaphylaxis for more than 1 day, we decided to exit persons at 1 day after start of anaphylaxis.
Submission 259 We submit one cohort definition that is currently in pending peer review status in OHDSI PL. id 259 and name ‘Anaphylaxis all cause’.
Cohort Definition Logic Description 259: we identify multiple events per person for anaphylaxis, i.e., one person may have more than one record. Because the median expected duration of anaphylaxis is 1day, we decided to define the end date of the phenotype after 1 day from the last date of continuous care for anaphylaxis. Because we opined that any further anaphylaxis within 30 days may reflect ongoing care for a prior anaphylaxis event, we used a collapse strategy that joined any new event to be part of the old event. For detailed logic please read human readable text on data.ohdsi.org/PhenotypeLibrary shiny application for cohort id 259.
Phenotype Evaluation 259:
- I observed that we have about 1.1 to 1.5 events per persons with 90%ile of person entries having time in cohort of 2 days. This suggests that our cohort exist strategy is correct.
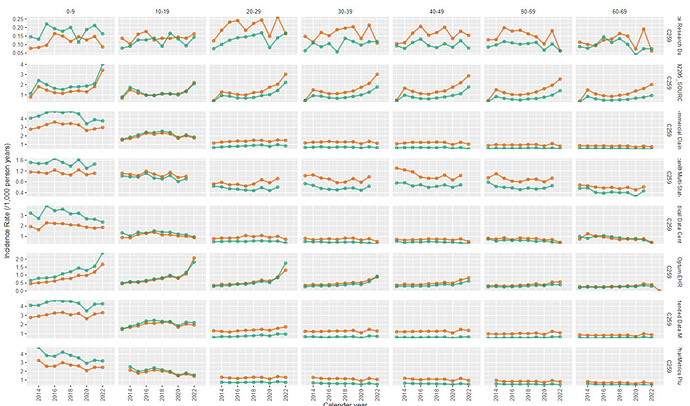
- On incidence rate plot, I observe most data sources to have similar incidence rate - with most differences explainable by data source heterogeneity all within an order of magnitude of each other. However, the data sources that had the lowest incidence rate appear to be those that do not have inpatient data capture. Younger age had higher incidence rate compared to the other age groups.
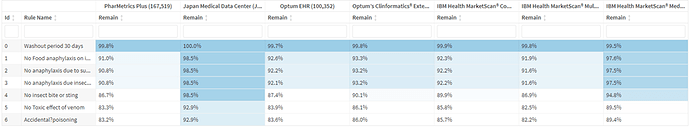
- On reviewing the index event breakdown, about 20% of persons appear to enter the cohort based on food anaphylaxis or toxin induced anaphylaxis.
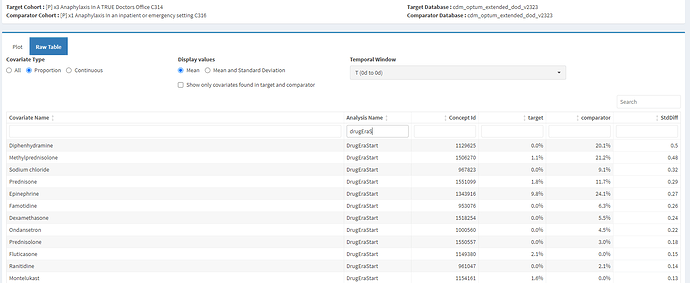
- More than 70% of visits that started on index date were outpatient visits. Because I expected that persons with anaphylaxis were being managed in emergency setting, this observation of people being managed in an outpatient or office setting made me think of specificity errors. However, when I reviewed the characterization of this cohort on Day 0 - I observe that more than 50% of persons have received epinephrine, along with other drugs commonly used to manage anaphylaxis like antihistamines, steroids. This allays my concern of specificity errors and suggests that these are true cases.
- In the time window immediately prior (-1 to -30d) I observed some use of drugs for the treatment of anaphylaxis - suggesting index date misclassification.
Cohort Definition id 221:
Justification for second cohort definition 221: I presented this, potentially conflicting diagnostics to my collaborators, specifically the presence of higher incidence in younger age groups, observation of outpatient visits and the utilization of drugs. Our collaborators focused the discussion on the finding that more than 20% of persons indexed on food anaphylaxis. After discussing we decided to explore if it was possible to phenotype a subset of anaphylaxis – that we decided to call “Anaphylaxis when a person was not known to be exposed to certain likely or known allergens” (a new phenotype), i.e. in this phenotype we are using a modified Sampson’s criteria as a case definition i.e… we want to remove persons who were reported to have anaphylaxis after an environment allergen (likely or known) e.g. bee stings. We decided to call this phenotype as ‘Anaphylaxis non environment exposure related’ and specifically indicate that persons with environmental exposure to a likely or known allergen would not meet this new case definition.
Submission 221: Based on the justification above, we submit the second cohort definition which is currently pending peer review in OHDSI PL and has the id 221 with the name ‘Anaphylaxis Non Environmental exposure related’.
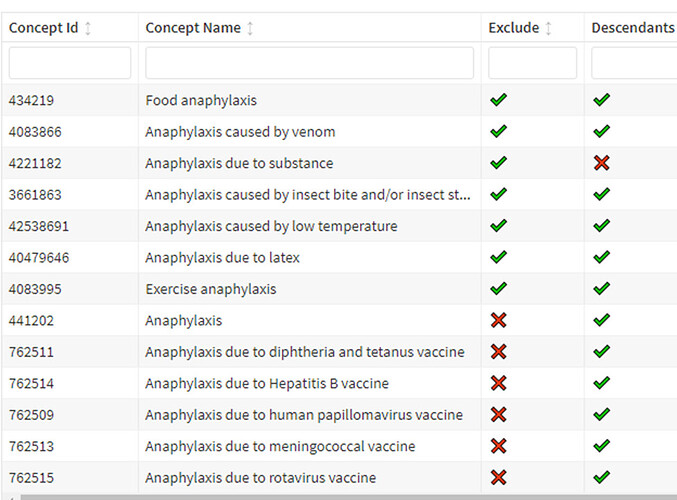
Logic Description 221: Compared to 259 (all cause) we allow persons to enter on a narrower set of concept set that excludes concepts related to food, and environmental exposure. In addition, to remove persons who may have both a more ‘non-specific’ anaphylaxis code and a specific anaphylaxis code, we added additional rules that remove events were persons are found to have food or environmental anaphylaxis.
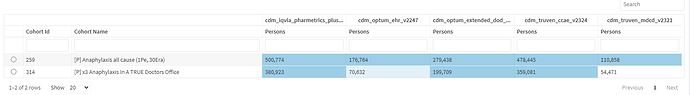
Phenotype Evaluation 221: Cohort counts: Compared to 221 (all causes), the new 259 (non-environment) had a considerable reduction in counts – sometimes more than 50%. This implied that persons with food and environmental anaphylaxis accounted for many persons in most data sources and it is important to evaluate this sub-type of anaphylaxis as its own phenotype.
Notice in the attrition table below – the use of inclusion rules was not the reason for this reduction in count.
It was mostly from persons not entering via the modified concept set above. i.e., a lot of persons with anaphylaxis are coded with only the food or environmental anaphylaxis concept, and the number of events/persons with both a non-specific anaphylaxis concept and a food/environmental concept at the same time is relatively small - only about 8%.
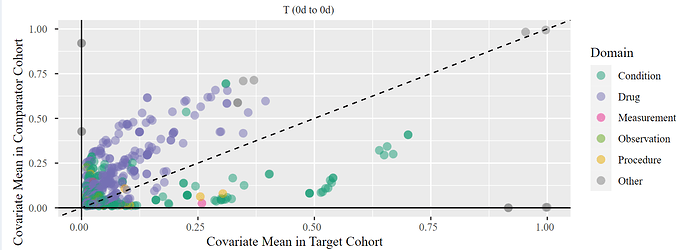
I then compared the cohort in 259 and 221 – and found the population level characteristics of treatments very similar on index date and in the period immediately after. However, there were differences in incidence rate with younger age group - the newer restrictive definition had the same incidence rate in all age groups, while the all anaphylaxis had higher incidence among younger ages. This is explained by prior knowledge that younger individuals are more likely to have food and environment related anaphylaxis compared to older adults.
Overall 259: We submit 259 to be a subset of 251 but a different phenotype (i.e. non environmental). On review of the treatment patterns, the characteristics of 259 is similar to 251, and so both cohorts have anaphylaxis.
Submission 258: Finally – we are submitting a third cohort definition. This is based on a previous OHDSI work discussed here Phenotype Phebruary Day 24 - Anaphylaxis as part of Phenotype Phebruary 2022. This cohort definition attempted to replicate the guidance from the Food and Drug Administration (FDA) and is detailed in the referenced post.This new cohort definition appears to capture most of the person in 258, but not all. But it also captures persons who are not in 258. Interestingly, unlike 221; 258 is trying to model all anaphylaxis and not attempting to avoid environmental allergen related anaphylaxis. It appears to have lower sensitivity compared to 259.
Overall: We have submitted three cohort definitions for the consideration for peer review. We expect 258 to have more sensitivity error compared to 259. We however argue that 221 (non-environmental) is attempting to model a slightly different clinical idea (phenotype) that we describe as “Anaphylaxis Non-Environmental exposure related”. We observe some evidence of index date misclassification in the -1 to -30d window that is residual and not corrected.