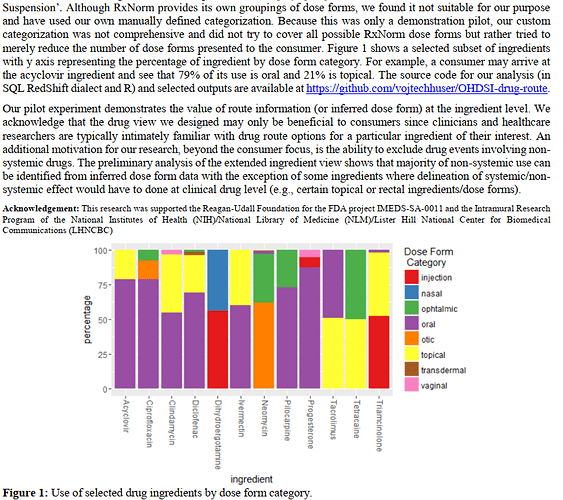
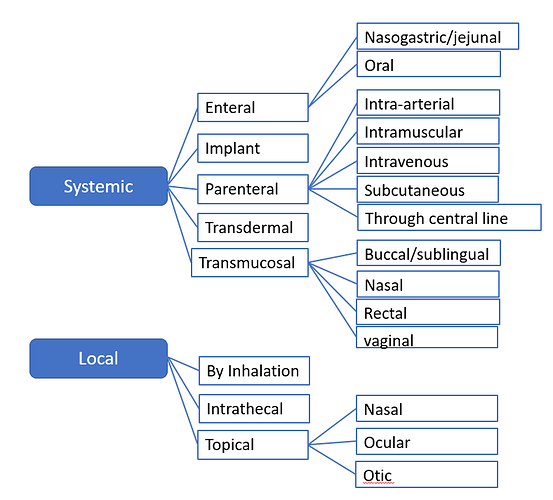
Hi guys,
I’ll just chime in because we’re building an ICU OMOP CDM in this period, and I’m personally working on (read: wrestling with) our drug data. Theresa’s approach seems to fit our use very well.
Drug use in the ICU is a bit funky with some weird, but crucial, edge cases that need to be handled correctly to obtain the insights we look for. In particular, dose form is not necessarily a good proxy for administration route, and concentrations can be weight-based (to obtain similar infusion rates in different patients even if they need different amounts of a given drugs; this is the case for e.g. norepinephrine which is used a lot in ICUs).
One interesting route-related challenges is vancomycin: in our data, vancomycin has been given via six different routes: intravenous, via nasogastric tube, inside the bladder, intraperitoneal, local (through a absces drain) and intrathecal. The nasogastric route is used only for clostridium difficile colitis because you want the drug to work in the intestinal lumen and, thus, do not want it to be absorbed.
I’d be happy to proven wrong, but as far as I’ve been able to gather from relationships in the CDM, the best way to do this is to map vancomycin to the ingredient (1707687) and denote the actual administration route in route_conept_id. If we isntead map to e.g. a clinical drug, analyses might try to infer the administration route through the relationships, which would be incorrect. At least, that’s what I’d worry about as per this:
WITH vanco_drugs AS (
SELECT *
FROM concept_relationship
WHERE concept_id_2 = 1707687
AND relationship_id = 'RxNorm has ing'
),
drug_forms_of_vanco AS (
SELECT cr.*
FROM concept_relationship AS cr
INNER JOIN vanco_drugs AS vd
ON vd.concept_id_1 = cr.concept_id_1
AND (
lower(cr.relationship_id) LIKE '%route'
OR lower(cr.relationship_id) LIKE '%form%'
)
)
SELECT
c1.concept_name AS drug_name,
dfv.relationship_id,
c2.concept_name AS dose_form_name
FROM drug_forms_of_vanco AS dfv
LEFT JOIN concept AS c1
ON c1.concept_id = dfv.concept_id_1
LEFT JOIN concept AS c2
ON c2.concept_id = dfv.concept_id_2
which yields:
| drug_name |
relationship_id |
dose_form_name |
| sodium chloride / vancomycin Injectable Solution |
RxNorm has dose form |
Injectable Solution |
| Vancomycin Oral Lozenge |
RxNorm has dose form |
Oral Lozenge |
| Vancomycin Ophthalmic Solution |
RxNorm has dose form |
Ophthalmic Solution |
| Vancomycin Ophthalmic Ointment |
RxNorm has dose form |
Ophthalmic Ointment |
| vancomycin Injectable Suspension |
RxNorm has dose form |
Injectable Suspension |
| vancomycin Injectable Product |
Has dose form group |
Injectable Product |
| vancomycin Oral Liquid Product |
Has dose form group |
Oral Liquid Product |
| vancomycin Oral Product |
Has dose form group |
Oral Product |
| vancomycin Pill |
Has dose form group |
Pill |
| Vancomycin Intravenous Solution |
RxNorm has dose form |
Intravenous Solution |
| Vancomycin Intravenous Solution |
RxNorm has dose form |
Intravenous Solution |
| Vancomycin Oral Powder |
RxNorm has dose form |
Oral Powder |
| vancomycin Injectable Solution |
RxNorm has dose form |
Injectable Solution |
| vancomycin Oral Capsule |
RxNorm has dose form |
Oral Capsule |
| vancomycin Oral Solution |
RxNorm has dose form |
Oral Solution |
| vancomycin Oral Suspension |
RxNorm has dose form |
Oral Suspension |
| vancomycin Injection |
RxNorm has dose form |
Injection |
| Vancomycin Topical Powder |
RxNorm has dose form |
Topical Powder |
| Gentamicin / heparin / Vancomycin Injectable Solution |
RxNorm has dose form |
Injectable Solution |
| Vancomycin Prefilled Syringe |
RxNorm has dose form |
Prefilled Syringe |
| Vancomycin Injectable Suspension |
RxNorm has dose form |
Injectable Suspension |
Again, this is the first time I actually work with mapping drugs, so I’d be happy to be proven wrong and would greatly appreciate some hints to places where I can reach a higher leve of understanding.
Cheers,
Ben