Attach cohort definitions: Because I am a new user, it will not let me upload a file into the submission. I have added the link to our cohort definition at the bottom of this post.
Cohort Definition Names: HSV Anterior Uveitis (SUN), HSV Anterior Uveitis (SUN sensitivity analysis)
Contributor names:
| Contributor | ORCID | Organization | |||
|---|---|---|---|---|---|
| Edward Lee | N/A | Roski Eye Institute, Keck School of Medicine, USC | |||
| Kiana Tavakoli | 0000-0003-1883-9018 | Shiley Eye Institute, University of California San Diego | |||
| Rupesh Agrawal | 0000-0002-6662-5850 | National Healthcare Group Eye Institute, Tan Tock Seng Hospital, Singapore | |||
| William Rojas Carabali | 0000-0002-9976-8989 | Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore | |||
| Karen Armbrust | 0000-0001-9381-4756 | Minneapolis VA Health Care System, University of Minnesota | |||
| Kareem Moussa | 0000-0001-9110-9594 | Department of Ophthalmology & Vision Science, University of California, Davis | |||
| Jessica Shantha | 0000-0002-4449-8598 | F.I. Proctor Foundation, University of California, San Francisco | |||
| Edmund Tsui | 0000-0001-7532-9191 | UCLA Stein Eye Institute, David Geffen School of Medicine at UCLA | |||
| Brian Toy | 0000-0002-9612-5697 | Roski Eye Institute, Keck School of Medicine, USC |
Clinical description: Computable definition of SUN classification criteria for HSV anterior uveitis. This phenotype is of patients with a diagnosis or clinical findings of anterior uveitis that is consistent with an etiology of herpes simplex virus (HSV) infection. This phenotype operationalizes the definition published by the SUN workgroup (Classification Criteria for Herpes Simplex Virus Anterior Uveitis - PubMed (nih.gov))
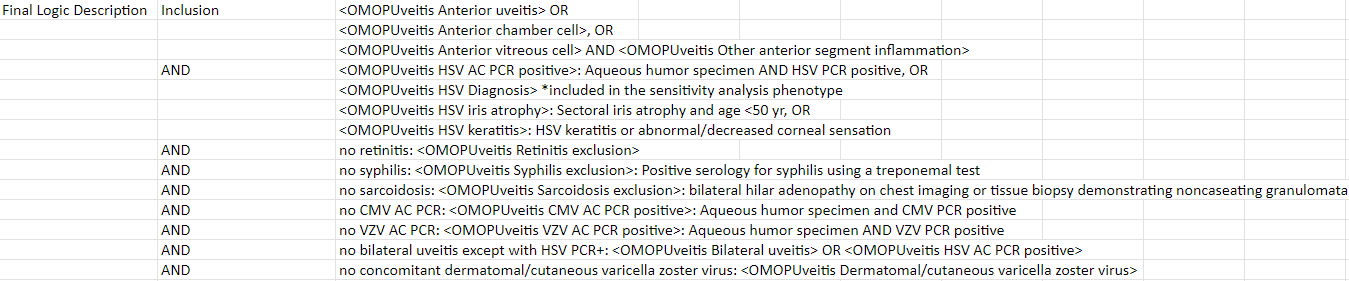
Logic description:
Inclusion
-
Evidence of anterior uveitis
a. anterior chamber cells
b. if anterior vitreous cells are present, severity is less than anterior chamber inflammation
c. no evidence of retinitis -
Unilateral uveitis (unless there is a positive aqueous PCR* for varicella zoster virus)
AND
-
Evidence of herpes simplex infection in the eye
a. aqueous humor PCR positive for herpes simplex virus OR
b. sectoral iris atrophy in a patient ≤50 years of age OR
c. herpes simplex keratitis
d. included in sensitivity analysis phenotype diagnosis of concurrent HSV infection
Exclusions
- Concomitant dermatomal/cutaneous varicella zoster virus (unless aqueous specimen PCR positive for herpes simplex virus)
- Positive serology for syphilis using a treponemal test
- Evidence of sarcoidosis (either bilateral hilar adenopathy on chest imaging or tissue biopsy demonstrating non-caseating granulomata)
- Aqueous specimen PCR positive for cytomegalovirus or varicella zoster virus
Final Logic Description used to construct phenotype on ATLAS:
Recommended Study applications: target
Submitted cohort definition: ATLAS: Cohort Definitions (ohdsi.org)
Submitted cohort definition: ATLAS: Cohort Definitions (ohdsi.org; sensitivity analysis)