Hello! My name is Chris Mecoli; a bit of background – I work as a rheumatologist at Johns Hopkins in our Myositis Center. I’m joined by Zach Wang (data science graduate student) and Will Kelly (database manager) who bring their data science expertise to this project. We are all relatively new to OHDSI, and are excited to increase our involvement. Thanks to @Gowtham_Rao et al for holding an interactive session to help review our phenotype. Now, on to the Phenotype of interest: Adult Dermatomyositis.
Adult Dermatomyositis
Develop a shared clinical understanding of the phenotype
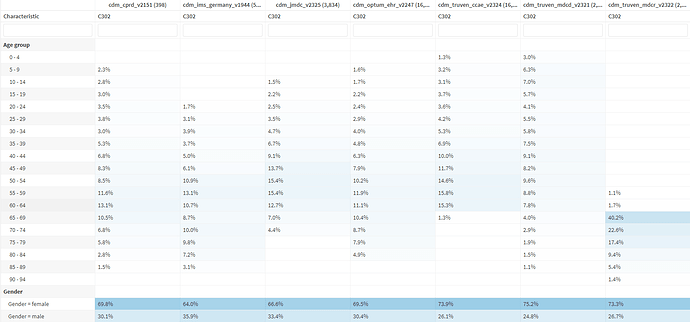
Adult Dermatomyositis (DM – not diabetes!) is a rare disease (estimated incidence 10 cases per million people) that causes muscle inflammation and skin rash. It’s one of a group of muscle diseases that cause muscle inflammation and swelling. It’s different from other muscle diseases because it also causes skin problems. Dermatomyositis is the term used to describe both muscle and skin symptoms. Because of the heterogeneity of this condition, often times many different providers/specialists will care for DM patients, including rheumatologists, neurologists, primary care physicians, pulmonologists, and dermatologists. DM can occur at any age, but it most often affects adults ages 50 to 70. Women are twice as likely as men to be diagnosed with the disease. The symptoms include rashes throughout the body, proximal muscle weakness, trouble swallowing (dysphagia), muscle pain (myalgia), difficulty breathing (dyspnea), and joint pains (polyarthralgia).
How is dermatomyositis diagnosed?
No single test can make the diagnosis of DM; rather, it is the entire clinical picture of history, physical examination, blood work and ancillary testing. The most common blood tests include the muscle enzyme ‘creatine kinase’ and myositis-specific autoantibodies (e.g. Jo1, SAE, Mi2). Additional studies performed often include an electromyelogram (EMG), muscle MRI, and skin or muscle biopsy.
How is dermatomyositis treated?
There’s no cure for the condition, but the symptoms can be managed. Essentially all patients receive some form of immunosuppression (drugs such as prednisone, methotrexate, mycophenolate, azathioprine rituximab, intravenous immunoglobulin, and several others).
What is the prognosis?
DM is often a chronic condition lasting many years, although in a minority of patients, drug-free clinical remission can be achieved. The majority of patients will require monitoring and long-term treatment with some form of immunosuppressive/immunomodulatory therapy.
Differential diagnosis
Often on the list of DM mimics includes other rheumatic diseases such as systemic lupus erythematosus and systemic sclerosis. In addition, dermatologic diseases such as atopic dermatitis (eczema) and psoriasis can mimic dermatomyositis. Lastly, other forms of myositis (polymyositis, immune-mediated necrotizing myopathies) are considered when evaluating a patient for DM.
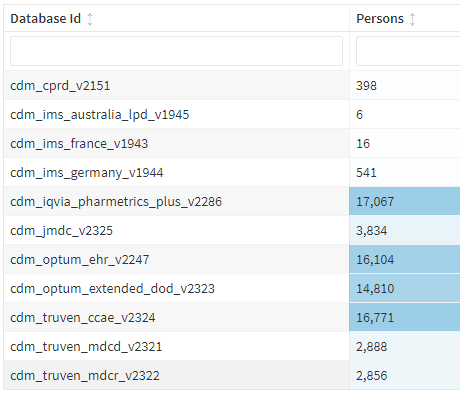
The goal of this phenotype is to study incident DM cases in any health care setting (inpatient, outpatient, ER).
Literature Review of DM in Observational Research
Several research groups have studied DM patients in observational research using diagnosis codes. The majority of these studies use ICD-9/ICD-10 codes for “dermatomyositis” – specifically codes 710.3 (ICD-9) and M33xxx (ICD-10). DM patients can be seen in any context (inpatient, ER, outpatient), and have been studied in all of these locations. Very few prior studies have incorporated other criteria beyond diagnosis codes (such as whether a specialist saw the patient, or whether the patient received corticosteroids/prednisone or underwent a muscle biopsy).
Pavon MR, Sanchez JE, Pescatore J, Edigin E, Richardson C, Manadan A. Reasons for Hospitalization and In-Hospital Mortality in Adults With Dermatomyositis and Polymyositis. J Clin Rheumatol. 2022 Mar 1;28(2):e433-e439. doi: 10.1097/RHU.0000000000001754. PMID: 34262001.
Kwa MC, Ardalan K, Laumann AE, Nardone B, West DP, Silverberg JI. Validation of International Classification of Diseases Codes for the Epidemiologic Study of Dermatomyositis. Arthritis Care Res (Hoboken). 2017 May;69(5):753-757. doi: 10.1002/acr.23010. PMID: 27564726.
Yafasova A, Diederichsen LP, Schou M, Sun G, Torp-Pedersen C, Gislason GH, Fosbøl EL, Køber L, Butt JH. Increased long-term risk of heart failure and other adverse cardiac outcomes in dermatomyositis and polymyositis: Insights from a nationwide cohort. J Intern Med. 2021 Sep;290(3):704-714. doi: 10.1111/joim.13309. Epub 2021 Jun 3. PMID: 34080737.
Dobloug C, Garen T, Bitter H, Stjärne J, Stenseth G, Grøvle L, Sem M, Gran JT, Molberg Ø. Prevalence and clinical characteristics of adult polymyositis and dermatomyositis; data from a large and unselected Norwegian cohort. Ann Rheum Dis. 2015 Aug;74(8):1551-6. doi: 10.1136/annrheumdis-2013-205127. Epub 2014 Apr 2. PMID: 24695011.
Smoyer-Tomic KE, Amato AA, Fernandes AW. Incidence and prevalence of idiopathic inflammatory myopathies among commercially insured, Medicare supplemental insured, and Medicaid enrolled populations: an administrative claims analysis. BMC Musculoskelet Disord. 2012 Jun 15;13:103. doi: 10.1186/1471-2474-13-103. PMID: 22703603; PMCID: PMC3480956.
Building a Cohort Set Expression
DM diagnostic and classification criteria exist that are considered the ‘gold standard’ for defining DM cohorts. The two most common diagnostic/classification criteria used in research are referred to as “Bohan and Peter” established in the 1970s and the ACR/EULAR 2017 Classification Criteria. However, the criteria in each are typically not available in most EHR or claims data sets (e.g. concepts such as “Gottron’s sign”, “proximal muscle weakness”, “elevated CPK levels”, and “myopathic findings on electromyogram” are rarely coded as such).
It is likely that only consortia/registries that specialize in Myositis will have this level of detail within their OMOP data (custom concepts would need to be created and mapped to OMOP standard vocabulary). For datasets that do not have this level of granularity, an alternative approach is needed. Therefore, we constructed two cohort definitions for adult DM patients, one to maximize sensitivity, one to maximize specificity, depending on the research question. These will be included in a follow-up post leading into the weekend.
 Given that the symptoms and treatments are both largely non-specific, I’ll be curious to see how the evaluation results play out, particularly if we’re able to run PheValuator
Given that the symptoms and treatments are both largely non-specific, I’ll be curious to see how the evaluation results play out, particularly if we’re able to run PheValuator