Target Clinical Description
- Pancreatitis Pancreatitis - StatPearls - NCBI Bookshelf 1
- Acute Pancreatitis Acute Pancreatitis - StatPearls - NCBI Bookshelf
- Chronic Pancreatitis Chronic Pancreatitis - StatPearls - NCBI Bookshelf
- Alcoholic Pancreatitis Alcoholic Pancreatitis - StatPearls - NCBI Bookshelf
Summary: Acute pancreatitis (AP) is an acute inflammatory process of the pancreas, suspected in patients with severe acute upper abdominal pain but requires biochemical (Serum lipase > 3x) or radiologic evidence to establish the diagnosis. Presentation: AP is categorized as mild (no organ failure or complications), moderately severe (transient organ failure/complication that resolves within 48 hours), or severe (persistent organ failure of ≥1 organ). AP is different from Chronic Pancreatitis (CP) which may be asymptomatic for long-periods, interspersed with abdominal pain. There are no diagnostic criteria for CP, and is a clinical judgment based on imaging studies, typical patient history and absorption tests. Management of AP is mostly done in an inpatient setting fluid replacement, pain control, nutrition management, and intravenous hydration. If a cause for acute pancreatitis is found, e.g., gall stones, ERCP/cholecystectomy should be performed. If associated with hypertriglyceridemia, an insulin drip may be administered. Epidemiology: AP annual incidence proportion is 600-700 cases per 100,000 people in the US. Reported incidence is around 5 to 35 per 100,000 persons per year. Prognosis: AP patients can fully recover. AP minimum median duration is 1-7 days, and the maximum median duration is 30 days. A new AP episode can independently reoccur in the same patient after recovery from a prior episode. Disqualifiers: Persons with CP should be considered to be ineligible to develop AP, even though they may have flares that mimic AP. Hereditary/congenital pancreatitis: these conditions are considered distinct clinical entities. Differential diagnoses: acute mesenteric ischemia, perforated viscus, intestinal obstruction, peptic ulcer disease, hepatitis, cholangitis, cholecystitis. Strengtheners: gallstones, alcohol use, drugs associated with AP. Complications resulting from AP include acute peripancreatic fluid collection, acute necrotic collections within 4-weeks of AP onset, portosplenomesenteric venous thrombosis, and systemic inflammatory response syndrome.
(Talley, Nicholas J., G. Richard Locke III, and Yuri A. Saito, eds. GI epidemiology. John Wiley & Sons, 2008.)
Designated Medical Event - MedDRA PT terms: Autoimmune pancreatitis, Ischaemic pancreatitis, Oedematous pancreatitis, Pancreatitis, Pancreatitis acute
Phenotype Development: We made design choices in the cohort definition based on the following reasons.
- Note: this phenotype was developed and evaluated as part of the OHDSI 2022 workshop led by Jamie Weaver @jweave17 .
- [Empirical Decision] Limit to inpatient and ER visit: During the initial phenotype development we observed individuals to have new onset acute pancreatitis during outpatient visit (as defined by visit_concept_id). Clinicians in the room expressed angst with this finding, as they expressed that it is extremely unlikely that persons with true/suspected acute pancreatitis would be managed exclusively in an outpatient setting. PheValuator provided evidence, that despite the use of Outpatint visit we had good PPV estimates. After the OHDSI symposium 2022 meeting, we reviewed the patient profiles of persons with acute pancreatitis diagnosis managed in outpatient setting - and found that several persons, although had the visit_concept_id of Outpatient Visit, had another event code for emergency visit (CPT4 or revenue code), while persons who had an outpatient visit without the corresponding CPT4 or revenue code received a follow-up care. This was determined by patient profile review using tool CohortExplorer https://github.com/ohdsi/cohortExplorer (not submitted). This provided some empirical data, via patient profile review, and strengthened the clinician expectation that the care should be in ED or Inpatient. So we decided to modify may enter if they are simultaneously in an inpatient or ER visit, or had a procedure code indicating that the care was emergent.
- [Design Choice] Chronic Pancreatitis: all persons with a history of chronic pancreatitis at any time on or before acute pancreatitis are not eligible. This decision was decided a-priori as part of the clinical description.
- [Design Choice] Hereditary Pancreatitis: persons with hereditary pancreatitis at any time are not eligible.
Submission:
- We submit the following cohort definition for peer review. See cohort id #251 in peer review pending status of the OHDSI Phenotype library ATLAS
- Cohort Definition Logic Description: we are identifying multiple events per person for acute pancreatitis, i.e., one person may have more than one record. But all these records should start (index on) an inpatient or emergency setting, they should have no history of chronic pancreatitis or hereditary or congenital pancreatitis. Because the median expected duration of AP is 1-7 days, we decided to define the end date of the phenotype after 7 days from the last date of continuous care. If a person then subsequently has acute pancreatitis within 180 days, we assume that to be a continuation of the previous acute pancreatitis. We start with a broad entry event criteria that is based on a Concept Set called ‘Pancreatitis’, but we require the co-occurrence of ‘Acute Pancreatitis’ concept set within 1 day. We believe this design allows for improving specificity while also improving index date misclassification (See below). For detailed logic please read human readable text on data.ohdsi.org/PhenotypeLibrary.
- These have been evaluated on 11 data sources. Cohort Diagnostics output is available on data.ohdsi.org/PhenotypeLibrary (see cohort id 251).
Phenotype evaluation Acute Pancreatitis (251):
- Impact of inpatient and ER restriction: this phenotype should be only studied in data sources that have good capture of care in inpatient or ER visit. Using this cohort definition on data sources with incomplete or poor capture of inpatient or ER visit is expected to lead to sensitivity errors. In the 11 data sources evaluated, we observed <5 counts in 5 data sources as those data sources are not expected to have inpatient or emergency room visit related data. In our patient profile review, persons who entered on a non-inpatient or ER setting (do not have the procedure code) appeared to be for follow-up of acute pancreatitis (not submitted).
- Impact of requiring ‘Acute Pancreatitis’ concept set: As described above in logic description, we allowed persons to enter based on ‘Pancreatitis’ and then restricted to those with ‘Acute pancreatitis. Removing this rule would increase by about 2% to < 5%.
- Impact of removal persons with Chronic Pancreatitis: Removal of this rule led to loss of about 5 to <10% persons. We (not reported here) studied the population level characteristics of persons with chronic pancreatitis and observed that they had different baseline characteristics (higher rates of abdominal pain, nausea, vomiting, ER utilization, alcoholism, chronic liver disease, diarrhea) suggesting they are a distinct phenotype. So, we expect this rule to increase specificity with minimal to no loss in sensitivity.
- Impact of removal of persons with hereditary pancreatitis: the numerical impact of this rule to sensitivity is minimal (<.1% loss). Persons with this phenotype appear very young (not reported here) - so we expect the use of this rule may improve specificity.
- Diagnostics Persons vs Events and Time In Cohort: we observe a rate of about 1.05 per persons. We don’t know if this is high or low, but find this to be consistent across the data sources evaluated. Time distribution diagnostic suggests that most persons, if they have a subsequent visit for acute pancreatitis have it within 180 days – as atleast 90% of persons have less than 30 days of cohort era. This indicates that our cohort exit strategy is reasonable.
- Diagnostics – incidence rate plot: We observe the incidence rate to increase with age decile with rates similar when stratified by sex. We observed an incidence rate that was about 10 times above reported, with highest rates in 60 to 69 age deciles.
- Diagnostics – index event breakdown: CohortDiagnostics index event breakdown diagnostic was not useful for this cohort, as it is reporting on the entry event concept set (which is of visit domain)
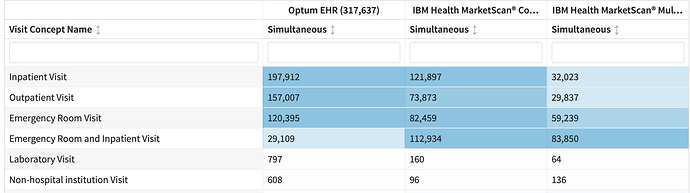
- Diagnostics – visit context: CohortDiagnostics visit context diagnostic was not informative as events are limited to inpatient and ER by design. However, we are observing a large number of persons who had outpatient visit starting simultaneous. These may be the persons who have the CPT4 codes for ER utilization.
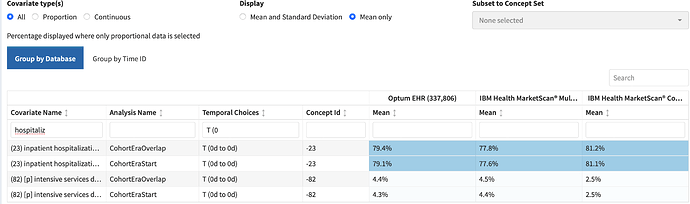
- Diagnostics – characterization: Overall the population level summary characteristics appeared consistent with persons with acute pancreatitis. Notably - ~ 50% had abdominal pain on day 0 , ~ 15% nausea vomiting , ~ 20% biliary calculus , ~ 7% alcoholism , ~ 8% dehydration , ~ 25% had lipase measurement , ~ 50% classified as emergency . There was also evidence of end organ failure commonly associated with acute pancreatitis such as acute renal failure. We observe high use
Summary of evidence on operating characteristics:
Evidence of sensitivity errors: We are observing the use of acute pancreatitis diagnosis codes in outpatient setting without inpatient or ER visit. Based on a limited review of patient profiles we opine that such persons are more likely to be outpatient follow-up of acute pancreatitis rather than new events. This opinion, if wrong, may indicate the presence of sensitivity error.
Evidence of specificity errors: I did not observe in characterization the presence of conditions that are either differential diagnosis of Acute pancreatitis such as mesenteric ischemia, ischemic colitis.
Evidence of index date misclassification errors: I observed in the –30d to –1d about 3 to 5% of persons to have acute pancreatitis diagnosis. These are persons who had acute pancreatitis in an outpatient setting and were admitted in next few days. There was also the presence of acute gastritis, epigastric pain which may indicate progression of subclinical acute pancreatitis being mis diagnosed in early-stages.
Overall we believe we have made sound design choices for this cohort definition. We expect the performance of this phenotype to have good operating characteristics of sensitivity, specificity and index date misclassification – and recommend the use of this cohort definition in studies as indications (target/comparator) or outcomes.