Hi team,
wow it’s day 18 already! and it’s me again @azzashoaibi and again using @Gowtham_Rao account.
Today, I would like to start a discussion about the phenotype- Multiple sclerosis. Some of the material discussed here is captured from a prior work by @Jill_Hardin and @rmakadia !
You all now learned from @Gowtham_Rao the importance of clinical description! so let’s start MS clinical description.
Clinical description:
you can find the following definition by the national institute of Neurological disorder and stroke, Multiple Sclerosis | National Institute of Neurological Disorders and Stroke
Overview :
An unpredictable disease of the central nervous system, multiple sclerosis (MS) can range from relatively benign to somewhat disabling to devastating, as communication between the brain and other parts of the body is disrupted. Many investigators believe MS to be an autoimmune disease – one in which the body, through its immune system, launches a defensive attack against its own tissues. In the case of MS, it is the nerve-insulating myelin that comes under assault. Such assaults may be linked to an unknown environmental trigger, perhaps a virus.
Most people experience their first symptoms of MS between the ages of 20 and 40 and it’s more common among females; the initial symptom of MS is often blurred or double vision, red-green color distortion, or even blindness in one eye. Most MS patients experience muscle weakness in their extremities and difficulty with coordination and balance. These symptoms may be severe enough to impair walking or even standing. In the worst cases, MS can produce partial or complete paralysis. Most people with MS also exhibit paresthesias, transitory abnormal sensory feelings such as numbness, prickling, or “pins and needles” sensations. Some may also experience pain. Speech impediments, tremors, and dizziness are other frequent complaints. Occasionally, people with MS have hearing loss. Approximately half of all people with MS experience cognitive impairments such as difficulties with concentration, attention, memory, and poor judgment, but such symptoms are usually mild and are frequently overlooked. Depression is another common feature of MS.
Diagnosis:
There is no specific test for MS. Doctors use a medical history, physical exam, neurological exam, MRI, and other tests to diagnose.
Prognosis:
A physician may diagnose MS in some patients soon after the onset of the illness. In others, however, doctors may not be able to readily identify the cause of the symptoms, leading to years of uncertainty and multiple diagnoses punctuated by baffling symptoms that mysteriously wax and wane. The vast majority of patients are mildly affected, but in the worst cases, MS can render a person unable to write, speak, or walk. MS is a disease with a natural tendency to remit spontaneously, for which there is no universally effective treatment.
Treatment:
Currently there is no cure for MS. Many individuals do well with no therapy at all, especially since many medications have serious side effects and some carry significant risks. Steroid drugs may be prescribed to treat acute symptoms of an attack, such as inflammation; they do not affect the course of the disease over time. Several drugs have been approved by the U.S. Food and Drug Administration (FDA) to treat one or more forms of multiple sclerosis, either by decreasing attack frequency and severity, treating relapses, or delaying disease progression. Some drugs are taken intravenously, some by infusion, and some oral. All drugs should be prescribed and closely monitored by specially trained physicians, as some medications have serious side effects. In March 2019 the FDA approved siponimod tablets taken orally by adults to treat relapsing- forms of MS. Beta interferon drugs have been shown to reduce the number of relapses (exacerbations) and may slow the progression of disease. FDA-approved beta interferon drugs for MS include Avonex, Betaseron, Extavia, and Refib. Monoclonal antibody drugs are designed to alter the immune system response to inflammation. Approved drugs include Ocrevus, Lemtrada, and Tysabri. Other drugs approved include Copaxone, Gilenya, Aubagio, and Tecfidera, all of which address relapsng forms of MS. An immunosuppressant treatment, Novantrone, is approved for the treatment of advanced or chronic MA. Ampyra can improve walking in individuals with MS.
Spasticity, which can occur either as a sustained stiffness caused by increased muscle tone or as spasms that come and go, is usually treated with muscle relaxants and tranquilizers such as baclofen, tizanidine, diazepam, clonazepam, and dantrolene. Physical therapy and exercise can help preserve remaining function, and individuals may find that various aids – such as foot braces, canes, and walkers – can help them remain independent and mobile. Avoiding excessive activity and avoiding heat are probably the most important measures patients can take to counter physiological fatigue. If psychological symptoms of fatigue such as depression or apathy are evident, antidepressant medications may help. Other drugs that may reduce fatigue in some, but not all, patients include Symmetrel and Cylert. Although improvement of optic symptoms usually occurs even without treatment, a short course of treatment with intravenous methylprednisolone (Solu-Medrol) followed by treatment with oral steroids is sometimes used.
Things to consider given the clinical description:
Given that MS is a disease with a natural tendency to remit spontaneously it is important to define what kind of MS patients we want to model?
a. Patients with a new and first ever onset of MS
b. Patients with replace MS
c. Patients with evidence of MS regardless of the stage or the status of their disease
For the rest of this post I will be attempting to model the easiest scenario: Patients with evidence of MS regardless of the stage or the status of their disease
Prior knowledge and literature review: here is a table summarize publications that reported on the validation of algorithms to detect MS in observational data
| Author | Citation | Codes used | Other requirements | Validation |
|---|---|---|---|---|
| Culpepper | J Rehabil Res Dev. 2006 Jan-Feb;43(1):17-24. | ICD-9-CM: 340 | ≥1 medical claim inpatient or outpatient; or receiving VA pension for MS; or prescribed an MS-specific DMA | algorithm had 92% agreement with chart review |
| Widdifield | Mult Scler. 2015 Jul;21(8):1045-54. | ICD-9-CM: 340; ICD-10: G35 | one hospitalisation (primary or secondary discharge diagnosis) or five physician billings over 2 years | sensitivity:84%; positive predictive value: 86%; specificity: 100%; NPV: 99.9% |
| Marrie | Can J Neurol Sci. 2013 Nov;40(6):824-31. | ICD-9-CM: 377.3, 323.82,323, 341.9,341.0, 341.9; 340, 341.0 ICD-10: H46, G37, G36.9, G37.8, G36, G35, G36.0 | ≥7 hospital or physician claims: sensitivity: 78%, specificity: 77%, PPV:95% | |
| ≥5 hospital or physician claims: PPV: 95% | ||||
| Marrie | Neurology. 2010 Feb 9;74(6):465-71. | ICD-9-CM: 377.3, 323.82,323, 341.9,341.0, 341.9; 340, 341.0 ICD-10: H46, G37, G36.9, G37.8, G36, G35, G36.0 | prescription claims for an interferon, glatiramer or natalizumab ; physician hospital or prescription claims | ≥3 medical contacts: PPV 77.0% NPV: 76.1% |
| Culpepper | Neurology. 2019 Mar 5; 92(10): e1016–e1028. | ICD-9 code 340 | The preferred algorithm required ≥3 MS-related claims from any combination of inpatient, outpatient, or DMT claims within a 1-year time period; | Sensitivity (86.6%–96.0%), specificity (66.7%–99.0%), positive predictive value (95.4%–99.0%) |
Things to consider given prior knowledge:
• Prior evidence suggest diagnosis code for MS are of poor PPV. Mainly due to the fact that MS diagnosis is based on deferential diagnose strategy and so the presence of the code may be for proposes of ruling-out and not a final confirmation of the presence of the disease
• Researchers attempted to improve the PPV of the diagnosis code by adding requirements such as an occurrence of multiple diagnosis in different dates, evidence of hospitalization and/or occurrence of related drugs prescription.
phenotype development:
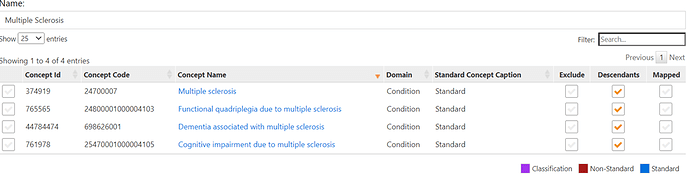
If you used phoebe to come up with initial code list, you will come us with the list below. The last 3 concepts were identified by PHEOBE
Now it’s time create a cohort definition. Relying on other’s work, I will model 3 cohorts. All three cohorts will use the same concept set but will vary in the logic as follow
| Cohort | Logic (entry event, inclusion criteria) | Exit criteria | Cohort in atlas |
|---|---|---|---|
| Culpepper 3x | Earliest occurrence of MS diagnosis, requiring ≥3 MS-related occurrences of any combination of inpatient or outpatient diagnosis, or S-specific disease-modifying therapies (DMT) within a 1-year time period; | End of observation period | ATLAS |
| Widdifield | Earliest occurrence of MS diagnosis, requiring 1 hospitalization with MS or 5+ occurrence of MS diagnosis in 2 year | End of observation period | ATLAS |
| Earliest occurrence of Multiple sclerosis | Earliest occurrence of MS diagnosis, | End of observation period | ATLAS |
*Note here that for simplicity we are modelling the exit criteria as the end of the observation period. However, such approach may not be proper given the clinical description of the disease. The clinical description of MS clearly state that patients can have an onset of MS but then the disease can go in remission and/or can also replace. The issue of modelling the most appropriate exist criteria is an evolving discussion that started in previous posts with @Gowtham_Rao , @Patrick_Ryan and others.
Phenotype evaluation
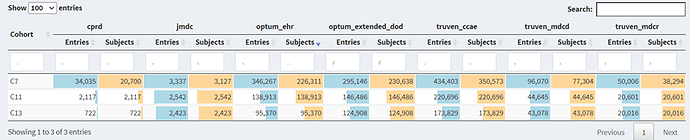
cohort count:
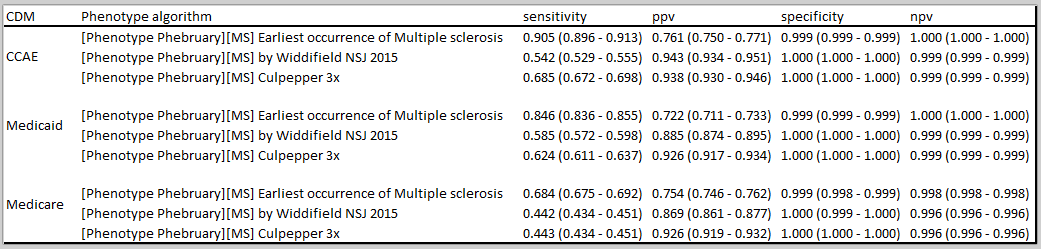
C7: Earliest occurrence of Multiple sclerosis (basic definition), C11: Culpepper 3x, C13: Widdifield
There is a significant drop in number of cases in both Culpepper (36%-46% in US data bases, 19% in JMDC, 90% in CPRD) and Widdifield (42%-56% in US data bases, 23% in JMDC, 97% in CPRD) when compared to the basic definition that has no restrictions. However, the drop in Culpepper approach is slightly less than what is observed in Widdifield (note that the sensitivity reported by Culpepper et al. is slightly higher than that reported in Widdifield et al paper).
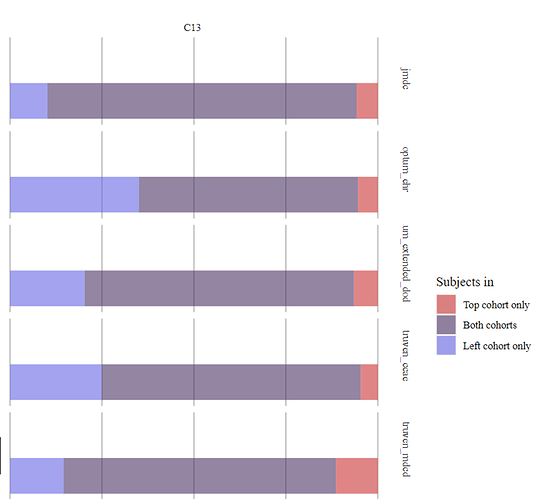
Cohort overlap—a significant overlap (60-84%) is observed in C11: Culpepper 3x, C13: Widdifield- Note that the two definitions only partially overlap by design.
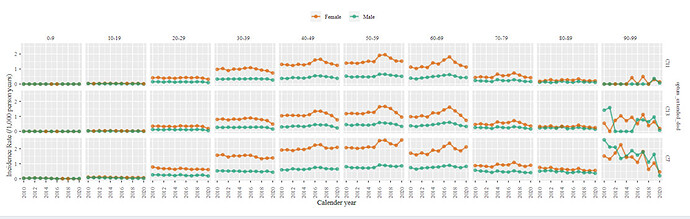
Incidence rate:
The clinical description state that MS patients first symptoms of MS occur between the ages of 20 and 40 and is more common among females. The incidence rate plot below is captured for MS among Optum DOD data. We can clearly observe a higher incidence among females and while the incidence do start to increase by age 20-26 an increasing incidence by age continues till 50. Indicating that we might be capturing some none incidence cases.
Temporal characterization:
At this point you all are familiar with this diagnostic. Based on the clinical description one can expect some of the following events to be observed among MS patients: muscle weakness, blurred vision, magnetic resonance imaging, MS drugs such as Glatiramer, Interferon beta and reoccurrence of MS code. The table below summarize the occurrence of some of these event among the 3 cohorts at different time in Optum DOD as an example
| Event | Time | C7: Earliest occurrence of Multiple sclerosis (basic definition), | C11: Culpepper 3x | C13: Widdifield |
|---|---|---|---|---|
| Muscle weakness | 30 days prior index | 1.8% | 1.5% | 2.1 |
| On index | 1.7% | 1.9% | 2.4 | |
| Blindness AND/OR vision impairment level | 30 days prior index | 0.3 | 0.4% | 0.4 |
| On index | 0.2 | 0.1% | 0.3 | |
| Brain MRI | 30 days prior index | 5.1 | 8% | 6.8 |
| On index | 8.4 | 8% | 7.1 | |
| glatiramer | 1 to 30 | 1.7 | 2.8% | 2.6 |
| 31 to 365 | 3.9 | 5.9% | 5.9 | |
| interferon | 1 to 30 | 3.2 | 6% | 4.6 |
| 31 to 365 | 6.5 | 10.6% | 8.5 | |
| Multiple sclerosis | 1 to 30 | 39.2 | 55.6% | 57.1 |
| 31 to 365 | 61.1 | 88.0% | 86.9 |
Given the significant overlap between patients in the 3 definitions, one should not expect much difference in occurrence of events among the 3 definitions. Hence, a small difference might indicate a meaningful improvement in specificity errors in one cohort versus the other. Directionally we observe higher occurrence of MS related events in C11 and C13 when compared to C7, maybe suggesting better specificity. Phevaluator @jswerdel will be a great tool to understand the tradeoff between specify and sensitivity here. Finally, many of the event related to MS (including drugs) are observed in the time periods before index-in all 3 cohorts. Such trend suggest that index event misclassification is possible in all 3 cohorts. Index date misclassification is likely since MS diagnosis involves ruling out other diseases so the exact date of diagnosis is variable in observational data.
Hope you found this useful ![]()