Just sharing with other researchers.
This article by Schneeweiss (always good to read any articles by that team btw).
https://www.researchgate.net/publication/380458311_Emulation_of_randomized_trials_of_direct_oral_anticoagulants_with_claims_data_and_implications_for_new_Factor_XI_inhibitors
has a great list of analyses where claims data was found fit for purpose.
I collect such analyses as a mechanism to testdrive a dataset on analysis where I have some estimate of what I expect to see.
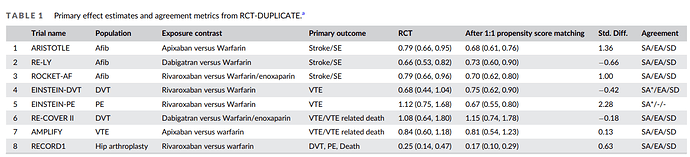
table of trials here:
A second nice set are all Darwin EMA studies (in recently published EMA catalog)
(link is using filter darwin=yes)
Do you have some other sources such that a researcher can arrive at a set of 300+ past questions where there is protocol published and indication that claims data was found as ‘fit for purpose’ ? (Note: regulatory grade !; initiated by FDA (or contract funded by them), EMA, etc.)