Hi all,
I’m trying to use the vocabulary to determine whether ICS drugs should be classified as low/medium/high dose medication for asthma. The amount of active substance is available in the drug_strength table, but what is classified as low/medium/high differs depending on the formulation used. See example table below:
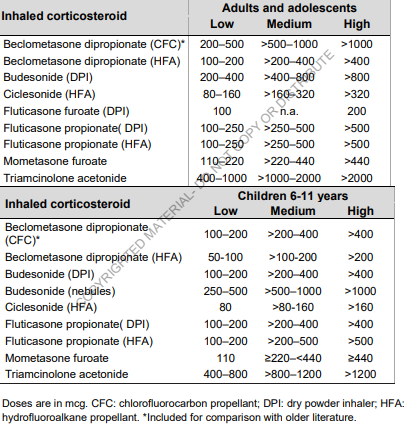
Examples of formulations are:
- CFC = chlorofluorocarbon propellant (not used anymore).
- HFA = hydrofluoroalkane propellant
- DPI = dry powder inhaler
- NEB = nebules
- UFA = ultra fine
- MDI = metered-dose inhaler
I want to link concepts at clinical drug level to these formulations to be able to use these different thresholds. I think dose form can help, but does not cover it entirely. Does anyone know if and how this could be done in OMOP CDM?
