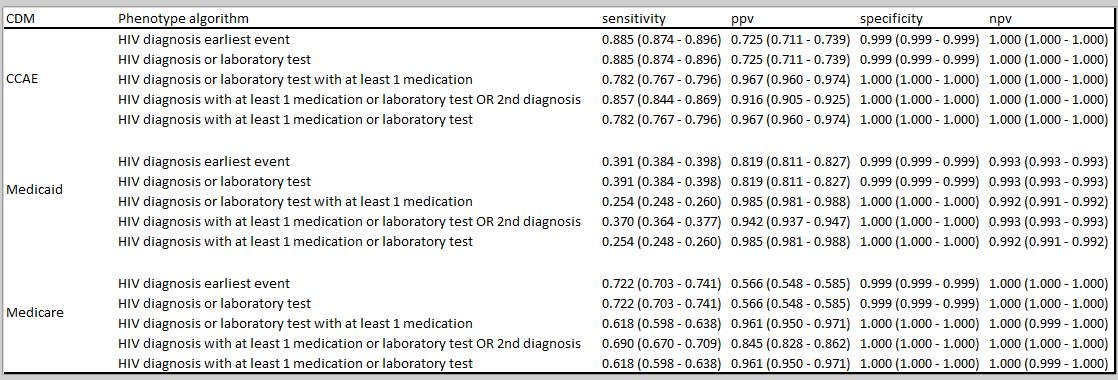
Here are the PheValuator results for HIV:
Several interesting things here.
- While it’s not surprising that “HIV diagnosis only” has the highest sensitivity, it was a little surprising to see that “HIV diagnosis or laboratory measure AND treatment OR 2nd diagnosis” only caused a small drop in sensitivity while producing a nice bump in PPV. This occurred across the 3 databases tested. That definition seems like a clear winner here. We normally see a significant drop in sensitivity when a second code is added (assuming the lab measure added little - see below).
- Surprised to see the large drop in sensitivity overall in Medicaid (those with lower SES) compared to CCAE (those generally under 65YO and working) and Medicare (those generally over 65YO). Anyone have any thoughts on why this might be (as @Patrick_Ryan might say - looking at you @rmakadia, @nigehughes and @Kevin_Haynes)?
- The numbers here are in line with the overall cohort counts indicating that adding a lab measure (HIV diagnosis or laboratory measure) doesn’t change the sensitivity. Similar findings for “HIV diagnosis or laboratory measure AND treatment” and “HIV diagnosis AND laboratory test OR treatment”.