Hi,
This forum topic is an attempt to get traction and consensus for a proposal to model treatment planning within the CDM.
Use-cases for modelling treatment plans:
1) Treatment compliance
Per forum thread Prescribed and Dispensed Drugs in the DRUG_EXPOSURE table the intention of the DRUG_EXPOSURE table is to capture the best evidence of a patient’s drug exposure.
As such, (generalising here) prescriptions are only intended to be captured in the case that there is no dispensation or administration data available (or whatever ETL rules are applicable in the target context to tease out what constitutes ‘best’ evidence), therefore it is not recommended to map both prescriptions and administration if both available, instead make a business rule that fits the context and select based on that. One consequence of this is that there is no obvious way to measure treatment compliance, as evidenced by the lengthy discussion.
2) Variation from prescribed treatment plans
This is the focus of my particular project, whereby we are setting out to measure unwarranted variation in cancer treatment. In order to do this, we need some way of modelling intended treatment protocols (centrally defined) in order to detect variation.
We will be focused on observing and characterising patterns of variation across cancer types (hypofractionation, modification to schedule, underdosing) and also detecting variation outliers.
We do not plan to model the acceptable variation parameters for each protocol (gotta draw the line somewhere!) but expect it to be a valuable undertaking to be able to specify treatment plans that encompass both chemotherapy (drug) and radiation (procedure) as prescribed, including dosage and schedule.
Any observed pattern of variation can be mapped back to the acceptable variation parameters at the cohort level, and outliers can be characterised from there.
We propose that it is possible to generalise the drug prescriptions table mentioned (but not decided upon) in the linked thread to capture prescriptions of both drugs and procedures.
3) Waiting lists / external delays to treatment
I wasn’t able to find anyone in the forum looking to study waiting lists or treatment delays, however it’s reasonable to expect that modelling care plans could be used in this way.
4) Adding granularity and structure to the ‘sig’ field in DRUG_EXPOSURE
At the moment this is just a string, but expanding the modelling to capture intention to treat would allow this to be structured as well.
5) Direct modelling of care plans
Per How to Capture Care Plan Data? this would allow for full description of care planning in the instance that the care plan itself may or may not be carried out.
Options:
There are a few options for modelling treatment intention.
-
Most simply, one could apply a modifier at the drug/procedure level, but as evidenced in the above discussion, there isn’t any real clarity around how this should be used in practice.
-
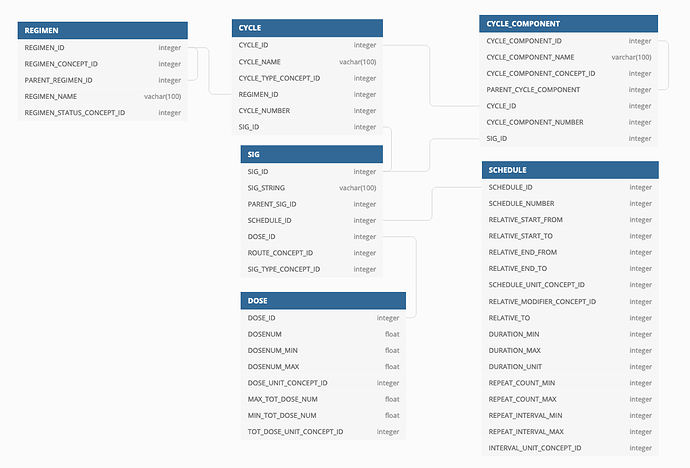
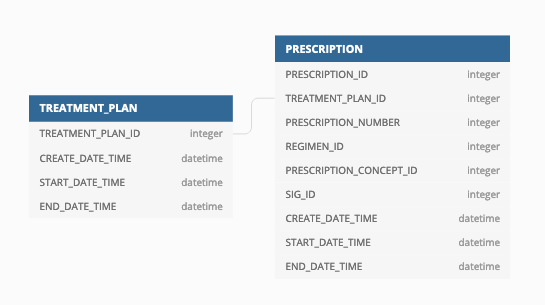
Create a new table that models nothing but intention to treat, perhaps TREATMENT_PLAN
The convention would therefore be formally and unequivocally state that DRUG_EXPOSURE and PROCEDURE_OCCURRENCE will hold the ‘best evidence of exposure’. This may be the same as a prescription record, or it may be dispensing administration, but will be defined as business rules in each context.
- Apply a modifier at the episode level. This would allow us to group treatment as planned and treatment as delivered into one place. The modifier could be added as a column in the EPISODE_EVENT table, where procedures and drugs below a level would then be marked as either observed or planned.
I suspect that options 1 and 3 may both end up with similar issues to the above discussion re: DRUG_EXPOSURE, where using the same structure for two purposes leads to heterogeneity in application and confusion, where option 2 has the additional benefits of explicitly modelling the SIG, and if TREATMENT_PLAN is linked under EPISODE_EVENT, the advantages of option 3 could still be achieved.
I’d love to chat with other people who have similar research questions, or other ideas about how they would want to use a potential TREATMENT_PLAN table and/or alternative solutions for the use-cases described above.
I have a proposal for how to model this data that I have piloted with various oncology treatment regimens, but I think this thread should open the discussion at a conceptual level first to see if there is indeed an appetite for it, before getting into the implementation weeds.
Thanks!