Thinking about consent and research infrastructure - I was researching informatics questions about deceased patients few years back. In this COVID time, it is tiny bit relevant perhaps. I know it is imperfect, but if we create a subset of COVID data from US that only contains deceased patients), than a special provision (in terms of IRB review [not needed]) for use of data on deceased for research apply. See details in this article here:
Title: Don’t take your EHR to heaven, donate it to science: legal and research policies for EHR post mortem
I applaud @Patrick_Ryan and community for this effort! As a physician researcher in women’s health, my team and I have some women specific research questions to propose with regard to pregnancy and lactation. There are guidelines from professional societies (ACOG, SMFMF’s joint statement here: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019) but there is not enough data to really inform these guidelines. Any additional clarity on these topics would be of tremendous public health interest.
~Pregnancy and lactation:
• rate of infection in pregnancy. By trimester if possible.
• complication rates for pregnant infected women
o severity of illness (versus non pregnant women, versus men)
o comorbidities
o length of illness
o transmission to newborn
o pregnancy complications: premature birth rates, fetal loss
• does cesarean section v vaginal delivery influence transmission to newborn?
• any transmission through breast milk to infant
I echo @SCYou’s earlier suggestion for analysis by sex for any and all research questions. There may be important sex differences that could drive different approaches to triage, diagnosis, and treatment. Additional research questions for women are listed below.
~Testing
• are women tested at the same rate? If not, why?
• Do women have access to testing?
~Clinical presentation
• Infection rates (known, will not be fully possible until after the epidemic and serology surveillance is conducted)
• Infections with and without symptoms, any difference when compared with men?
• Prevalence of presenting symptoms (not just respiratory)
• Severity of illness
• Length of illness
• Time from symptoms to hospitalization_and Impact of comorbidities
• Radiology findings – CT, CXR – any differences between men and women? _
• “silent spreaders” – any difference in rates between men and women?
~Health outcomes:
- Response to specific medications/treatments ie antivirals, and to vaccines when testing begins
• Required hospitalization
• ICU admissions
• Ventilator requirement
• Length of stay
• Death rates?
• Impact of comorbidities on sequelae of infection ie co-infection- flu , bacterial infections
- what is impact of COVID-19 on existing co-morbidities
~Social impact:
• Transmission rates-woman exposed versus man exposed. There may be differences in how women come into contact w virus compared with men. Ie women are more likely to take public transportation than men. Women are more likely in supermarkets.
• Differences in access to care
• Engagement of women versus men in healthcare related to outbreak, Women are more frequently front line health care workers (nurses, phlebotomists, aids, etc) so may experience more occupational exposure than men
~Can a Risk prediction model be developed for men and women?
o severity of illness
o need for ICU
o need for ventilatory support
o increased length of stay
Thank you all for your consideration!
I agree as well.
And what are the effects on the fetus or newborn baby ?
so many things need to be looked in the case of mother and newborn baby .
@ru-cheng @rajeev1 Great to hear this interest. We tried to set up the maternal and child health group in OHDSI some time ago, but there was minimal interest back then. In the IMI funded ConcePTION consortium https://www.imi-conception.eu we have developed a protocol for studies responding to these questions and it would be great to explore how to all collaborate. As we know pregnancy outcomes take some time to reveal especially if we wish to look at the impact on different trimesters, good thing: that buys us some time. Happy to organise a meeting to discuss.
I probably missed that, but do we have already a post looking into the role of co-infection with other viruses in more severe cases? I was thinking reactivated sleeping viruses such as Varicella Zoster or Epstein Barr. Varicella Zoster could be consistent with liver damage and the fact that children experience a severe course of disease less often, maybe because they had been routinely vaccinated.
I feel it would be good to review records of childhood diseases, vaccination status as well as looking into endemic prevalence of common viruses in hot spots that have a higher count of severe cases. With a quick lookup for VZV I found only this. Here Italy has a slightly higher incidence of cases in the age group 40+
Thanks @Miriam2! I think J&J has a representative participating in the IMI ConcePTION consortium already. I will be contributing to the input through our designated representative. But welcome to discuss in the context of OHDSI also.
Would anyone be interested in testing the hypothesis that beta-blockers are protective against morbidity and mortality from COVID-19 infection? There is some evidence that a catecholamine surge could be contribute to morbidity and mortality from COVID-19
- According to case reports, a significant proportion of COVID-19 patients develop arrhythmias during the hospital course. Is it possible that catecholamines are contributing to the onset of ventricular or supraventricular tachycardias?
- There is a perceived association between ACE inhibitor use and adverse COVID-19 outcomes. One proposed hypothesis is a synergy between ACE inhibitor use and viral infection. However, I wonder if this association is observed because ACE inhibitors are given instead of beta blockers and aren’t as protective against high levels of catecholamines.
- During the study-a-thon, I read a prediction model that suggested that pulmonary hypertension, rheumatic heart disease, and Takotsubo cardiomyopathy are protective against COVID-19 morbidity and mortality. Beta blockers can be a treatment for all of these conditions.
We could characterize what proportion of hospitalized COVID-19 patients, who develop adverse outcomes, had a history of beta blocker use. We could also characterize what proportion of hospitalized COVID-19 patients develop a new onset supraventricular or ventricular tachycardia. All feedback is welcome!
Thanks,
Matt
The ACE/ARB team in the Study-a-Thon could possibly take this on and you can join. @msuchard led that team. Comparator groups already were calcium channel blockers and thiazides. They already worked out the phenotype definitions for prevalent usage of drugs in hypertensive patients
Thank OHDSI Community! It was an honor to witness the Study-a-thon last weekend. With the current up-trending of COVID-19 with local transmission, the medication use would be expected to be demanded. Here are my suggestions. As a slow follower of the thread of discussion, if the topics have been mentions already, please accept my apologies.
-
Effect of early vs late initiation of lopinavir/ritonavir
: Lopinavir/ritonavir have been preferred in Korea and hydroxychloroquine is rarely prescribed until the study results by Cao et al (n=199, open-label). However, the potential benefit of the early use of lopinavir/ritonavir is mentioned. Could it be worthwhile to run a study with global data before we set aside one treatment option? https://www.ncbi.nlm.nih.gov/pubmed/32187464 -
Mortality, LOS, ICU stay by the duration of anti-COVID drug exposure.
: When to stop medications is always questionable. Would there be a better answer than continue them until two negative culture results out, per se? In ongoing clinical trials, intervention arms usually have 5~14 days of medication exposure periods, but it may be different in reality. -
Safety of hydroxychloroquine (or chloroquine) in arrhythmia, ventricular arrhythmia, QT-prolongation.
: As mentioned above by Matt Spotnitz, these complications could be due to catecholamine, concomitant CVD drugs. Or they could be due to the adverse reactions of hydroxychloroquine or maybe by the contribution of all. (no RCT for this, yet). -
Impact of lopinavir/ritonavir on treatment outcomes (such as mortality, opportunistic infections, relapse…) in patients with HIV (or HCV).
: Lopinavir/ritonavir tends to reduce the efficacy of other antivirals when used concomitantly due to CYP metabolism. Can HIV (or HCV) patients be compared in those with lopinavir/ritonavir to those without lopinavir/ritonavir? Or compare pre- and post?
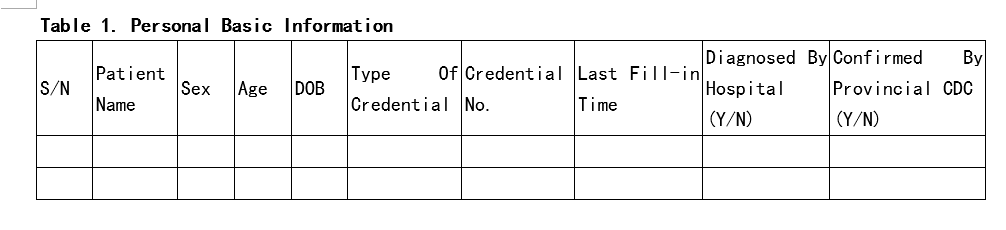
Hi, here is the data model we were/are collecting patients information fight the COVID-19. Considering the COVID-19 situtation in China, I think it’s definitely professinal and efficacious:) Wish could help friends here @Patrick_Ryan . Wish OHDSI could help the earth back to normal soon.COVID-19-data Model.docx (21.8 KB)
Thanks for posting. 50%+ can easily transform into OMOP.
The “intubation era” and the “tracheostomy” era is very interesting construct to track. We should think about how to put it into OMOP. (see picture below)
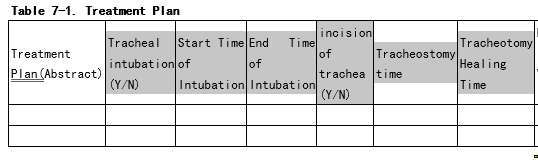
Are you considering re-ETLing the data or also collapsing existing records? If the latter, then how would you define the criteria to collapse records? For example, will a fixed interval (e.g. 5 days) will be enough? Or should we also look into visit_occurrence? For example, we have a scenario: a patient with ICD9 code 96.72 Continuous invasive mechanical ventilation for 96 consecutive hours or more has two records with an interval of 6 days, two distinct inpatient visits. Would it be considered as an era? What if the interval is 10 days?
I’m brand new to the community, and have probably missed such suggestions, but
- What is the overall effect of tocilizumab on the outcome of respiratory distress syndrome in COVID-19? Some clinical trials are in progress.
- What is the effect of intravenous immunoglobulin on the outcome of COVID-19?
There are some papers, that mention IVIG like
https://academic.oup.com/ofid/advance-article/doi/10.1093/ofid/ofaa102/5810740
Thanks,
Yuri
Hi Yuri, I saw your intro in the intro thread. You said you had experience / interest in cardiac risk factor analysis for various drugs, you say cancer drugs, but maybe you can set your interest in cancer aside for a moment and see if you could perhaps review and assess what’s known of the QT elongation issues with the following drugs:
- hydroxychloroquine
- azithromycin (there is some controversy, it might in reality actually be protective rather than independently aggravating with hydroxychloroquine)
- ivermectin at high dose
- favipiravir
- … add whatever other antiviral you want, remdesivir, ritonavir, etc.
The point of such review and comparative analysis would be to inform which of these candidates could be released as OTC drug into the wild, for early intervention / secondary prevention. My goal being almost exclusively the development of a drug to eradicate the pandemic long before any of the vaccines are available.
This is pretty close to the WHO CRF for COVID-19 (https://www.who.int/docs/default-source/coronaviruse/who-ncov-crf.pdf). Indeed it’s possible to transform part of this into OMOP @Vojtech_Huser, however there is very little date information, so I wonder what kind of analyses we could run it on. Perhaps some characterization studies? I don’t see how estimation studies could be run on the basis of this. But, if we could devise a few protocols that would make sense to run in these CRF’s, then there is a lot of global data that we could feed into it!
Is there an opportunity to design an observational study to look at whether any of the HIV treatment regimens offer protection against SARS-CoV-2 infection? Some of the newer compounds have been in Clinical Trials throughout the world to treat the pandemic. However, with large outbreaks such as the one that is still underway in New York, might lead to data on this research question assuming one can link drug therapy to illness. Emtricitabine/tenofovir is given as a prophylactic for HIV infection and might be a case control against those who have HIV and are on medication to control the disease.
A number of these HIV drugs have been identified via in silico screening and while it is probably late in the game to put new compounds in clinical trials observational work can still be designed and carried out. this is just a thought from a humble drug regulatory person.
As relatively recent research has shown that opioid use is associated with a substantial increase in risk of acquiring pneumonia,[1] it may be useful to integrate opioid exposure (especially moderate and high dose exposure) into the predictive models.
I’m aware of at least one other OHDSI study by Burn et al that reported on opioid use. Perhaps the existing code used to identify and quantify opioid use from this earlier project could be re-purposed for covid-relevant projects.
As I described in this thread a key goal should be to see if existing medications could be curative or protective against COVID-19: “Big data search for effective medications”
Are those Korean data sets for each patient detailed enough to determine which medications the COVID-19 patients were and were not taking at the time of their diagnosis?
Robert Clark
@Vojtech_Huser
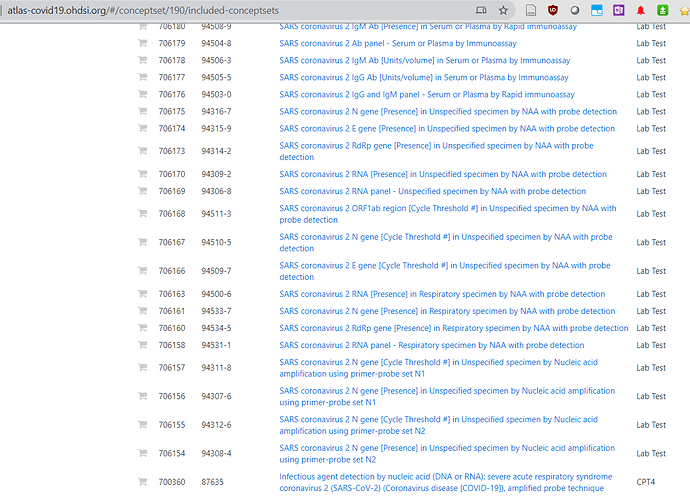
hello Vojtech - has there been any work on the mapping of the SARS-CoV-2 testing results LOINC codes to OMOP Concept IDs? WHere do I go to find that? Lisa
The temp LOINC codes have been assigned a CID (concept_id).
http://atlas-covid19.ohdsi.org/#/conceptset/190/included-conceptsets
They are not decomposed to LOINC parts since they are temporary and that will happen with regular release.
see