+1 for interest on this topic too.
The Health Plan for whom I work has looked at using the RIOSORD screening tool to help assess risk of overdose. It is a good start for triaging our members and targeting outreach, but I expect that it could be improved.
Does anyone in the OHDSI community recommend alternate screening tools or approaches for PLP?
Is the community interested in replicating and extending that study (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5939826/#pnx009-B19) to generate a more accurate and actionable PLP model?
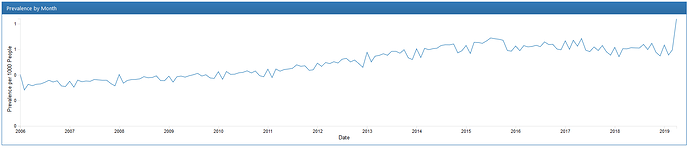
The instrument is a good start, but might benefit from being updated due to the age of the data used (only up through 2013), the lack of some important covariates (such as prescription for naloxone or treatment with metadone/suboxone; plus Social Determinants of Health in general), lack of clarity on exit conditions for the cohort, and relatively small size of the comparison cohort for their propensity matching.
We are just starting our OMOP journey (e.g. have not mapped our data yet), but we could help in the study design specification and evaluation if it could be run on others’ data that has already been mapped.