Thanks @rchen. I appreciate the vantage point of looking the clinical guidelines as a source of inspiration for where we can make important contributions. It seems there’s areas of opportunities for determining which recommendations may exist which are not fully supported by high-quality evidence (or could be further augmented with real-world observational studies), and also I’ve seen several areas in guidelines where recommendations cannot be made because of the lack of availability of any evidence to support alternatives. It seems the effect of fibrates to supplement statin use in reducing cardiovascular risk should be better understood if its going to be part of recommendations for how to handle specific subpopulations with uncontrolled hyperlipidemia. I’ll add this to our ballot so we can see how the rest of the community feels about it.
Thanks @BridgetWang. I’m very excited by this question. There is an ongoing RCT that is scheduled to report out next year to address this question (https://clinicaltrials.gov/ct2/show/NCT02092467), so OHDSI has an opportunity to ‘predict’ the RCT results using our observational data network, which has important methodological value for demonstrating the potential role of real-world data to augment, and in some circumstances, replace the need for RCTs, as has been proposed for consideration by the FDA and as part of the 21st Century Cures Act. The trial involves an active comparator, so we can define target and comparator cohorts for these drugs quite easily. Also, both trial outcomes (cancer, MACE) should be observable in most claims and EHR systems, as are most of the study inclusion criteria. There seems to be a lot of uncertainty about the safety of biologics in RA even after a lot of small clinical trials, so examining these outcomes in the real-world clinical practice across the OHDSI network could be an important contribution to the field.
Let’s see if the community is as excited as I am to pursue this question, I’ll add it to the ballot.
Agree. Thanks @Patrick_Ryan or the link to the Clinical trial. Just wanted to share a thought on why I think this will be a cool study.
From the molecular perspective, the TNF is one of the most notorious genes. Its promotor region on chromosome 6 has a plethora of variations leading to all sorts of disorder. In addition, a recent population level (kind of) exome sequencing of ~60,706 humans suggest variations in the enhancer regions of TNF that might contribute to dysregulation at the transcriptional level http://exac.broadinstitute.org/gene/ENSG00000232810. Our understanding of variations is generally gleaned by sequencing the patients with diseases - but not by how well or poorly they response (or would have responded) to a treatment.
OHDSI network study (across the massive datasets we have), therefore, might shed some light on the nature of patients according to their response to TNF inhibitors. Since we’ll know the features (comorbidities, drug etc) associated with such patients who do or do not respond to TNF inhibitors, therefore it might guide us (in future) on what type of patients one should sequence to look for variations and disease biology.
Thanks @Patrick_Ryan . I am inclined to agree with you with the one caveat that its unusual for individuals to have a prolonged OTC exposure to PPIs without ever having it prescribed at some point. This might impact measurement of total exposure but there are ways we could overcome this. Looking at an era effect before they were available OTC might be one potential option.
The other thing that I want to emphasize when considering studies, is the potential impact for change in practice. CKD now affects 1 in 7 adults in the United States - and is increasing in prevalence across the globe. The etiology of the overwhelming majority of cases is not identified. Further PPI use is becoming increasingly more prevalent worldwide and being able to quantify a relationship here would have important public health implications globally. There is perhaps a subset of patients with a significantly higher risk where PPI avoidance would be particularly important.
In considering the F-2-F choices, with the 1st proposal we would ideally need pathological stage (to reduce variation we may consider only looking at stage 1 or localized disease). At the VA that data is not currently in the OMOP CDM but we could link back to it from the CDW. Doing this at the meeting; however, would likely be problematic due to the VA’s data security (at least for me). I’m wondering who else have cancer stage in their OMOP data.
Stephen
Response from Guy:
Thank you for raising this question.
As for all maintenance treatments for chronic diseases, also in asthma patients, non-adherence (non-compliance) is an important problem.
Therefore, dispensing records from pharmacy systems or prescriptions written in EHRs are only proxies for exposure (no guarantee of exposure, and appropriate use / correct inhalation).
Dispensing records are somewhat better than prescriptions.
Additional response from Katia:
With regard to adequacy of capture of death information: We are interested in all cause mortality and not necessarily death due to asthma (exacerbation) as this is indeed not available in all databases;
With regard to exposure and potential non-adherence or switching: There is the potential of underreporting of exposure as we only use prescription or dispensing data however this would not necessarily imply that it would be differential between LAMA (without ICS) vs LAMA+ICS. Also we could try to control for eventual bias in adherence by calculating adherence at time of study start (for instance MPR in previous year) and match on MPR.
it’s COPD stage C or stage D
Right. Since those stages imply that patients have significant complaints, I’ll be surprised if they can walk out of clinic without a LABA prescription.
@rimma The Oncology Workgroup has pending proposal to place pathological staging in a new CANCER_MODIFIER table. The idea is to create a more narrowly scoped EAV structure dedicated to the observation/measurement heavy domain of Oncology. And for the choose-your-own-adventure conundrum of Oncology vocabulary choices to be eliminated by relying on an effort called the Nebraska Lexicon to encode the College of American Pathologists Cancer Protocols/Checklists in SNOMED (attribute) and LOINC (value) pairs.
See here: https://www.unmc.edu/pathology/informatics/tdc. We are actively looking for people trying to fit Oncology data into the CDM to vet the proposal with real data. We anticipated that much pathology data would need to be NLP-derived because so few institutions have discretely captured pathology data. See the proposal here: http://www.ohdsi.org/web/wiki/lib/exe/fetch.php?media=documentation:oncology:cancer_diagnostic_features_2018-02-07.pptx
The CANCER_MODIFIER table will likely be a near mirror of the current MEASURMENT table, but hang off of a CONDITION_OCCURRENCE or CONDITION_ERA, to further refine a cancer diagnosis.
As discussed on Tuesday’s call, I am excited to announce that
@BridgetWang’s research question has been selected by the community as our
target problem that we will collaboratively explore during our OHDSI F2F
next week. So everyone who’s coming: get ready to learn about
rheumatology! We’ll be evaluating the comparative safety of tofacitinib
vs. adalimumab and etanercept in patients with rheumatoid arthritis. Key
safety outcomes of interest include: malignancy, MACE, opportunistic
infections, hepatic evidence, and other non-MACE cardiovascular events.
We’ll be trying to replicate the design of an ongoing Phase 4 clinical
trial (Safety Study Of Tofacitinib Versus Tumor Necrosis Factor (TNF) Inhibitor In Subjects With Rheumatoid Arthritis - Full Text View - ClinicalTrials.gov), which is scheduled
to report out mid-2019. So, not only does this seem like a clinically
relevant and important question where we can provide real-world evidence to
inform current practice, but it also has important methodological
implications as a candidate demonstration of where observational data
analysis may be able to provide sufficient evidence to potentially supplant
the need for prospective investigations (either Phase 4 trials or
registry), if we can show that we can get comparable evidence faster, with
a larger population, and consuming less resources.
We had many great questions brought forth by the community on this thread,
and it was fun to see a lot of enthusiasm for several different topics. In
the interest in continuing to promote transparency, I thought it useful to
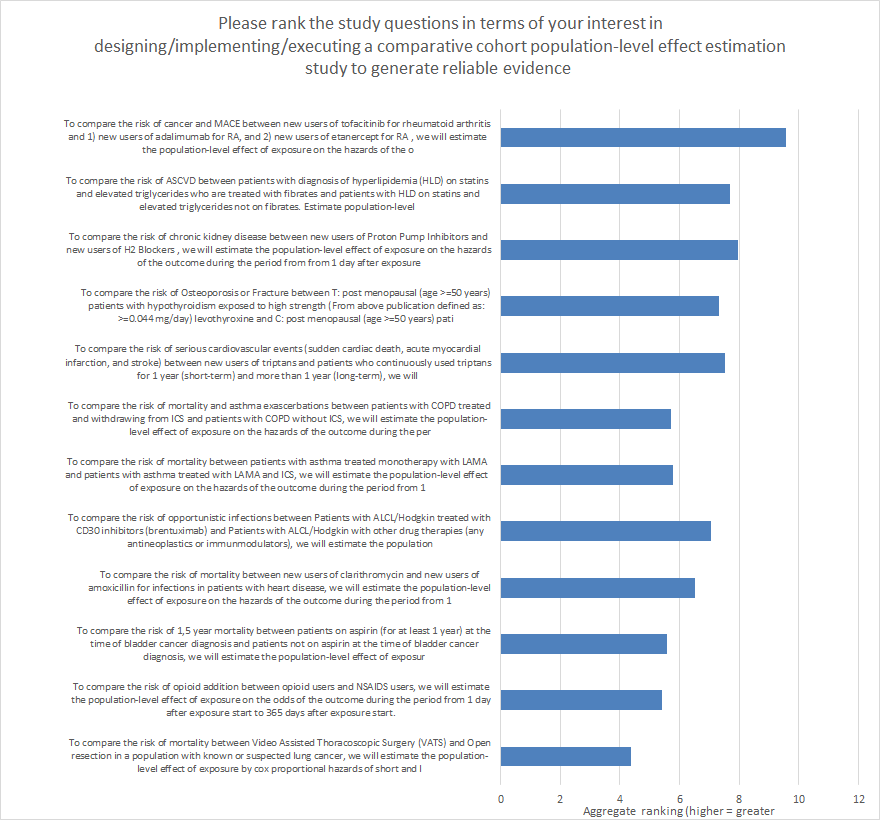
share the voting results. A majority of the OHDSI F2F participants did
submit a vote (35/60), and of the responses, @BridgetWang’s question had
the most 1st place votes (n=16) and the highest average score (9.5 on scale
from 1-12). Here’s a graph of the scores for all 12 questions that were
considered on the ballot:
After the OHDSI F2F, I do hope most, if not all of these questions will
also become network studies that we can all contribute and benefit from.
But for now, I’m looking forward to coming up to speed on @BridgetWang’s
research interest, and seeing how far we can get as a community in 2 days
when we work together to translate a question from idea to study design to
protocol to implemented code to executed network study to synthesized
evidence. This will be a very fun exercise, see many of you at Columbia
next week!
Hi all,
Could Bridget and others suggest background reading we might want to do
prior to the F2F so we better understand the clinical context and related
work?
Cheers,
David
As requested by @dsontag , @BridgetWang and I have put together a little homework for everyone attending the OHDSI F2F, as pre-reading to help you get up to speed on the clinical problem we’ll be collaborating on for two days:
Here is the link to the registration of the Phase 4 randomized clinical trial which we are trying to replicate the design and predict its outcome estimates:
NCT02092467 : https://clinicaltrials.gov/ct2/show/NCT02092467
Here are the links to the US Product labels for the three products we will study:
tofacitinib (Xeljanz): https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cf74ba2f-afc5-4baa-8594-979c889a5831
adalimumab (Humira) : https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=608d4f0d-b19f-46d3-749a-7159aa5f933d
etanercept (Enbrel) : https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a002b40c-097d-47a5-957f-7a7b1807af7f
Here’s links to four publications we think you might find useful as background context (ordered in priority in case you can’t get through all of the by Wednesday):
-
Ramiro et al. “Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis” Ann Rheum Dis. 2017 Jun;76(6):1101-1136. doi: 10.1136/annrheumdis-2016-210708: http://ard.bmj.com/content/76/6/1101.long
-
Machado et al. “Effectiveness and safety of tofacitinib in rheumatoid arthritis: a cohort study.” Arthritis Res Ther. 2018 Mar 23;20(1):60. doi: 10.1186/s13075-018-1539-6.: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5865387/
-
Cohen et al. “Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials” Ann Rheum Dis. 2017 Jul;76(7):1253-1262. doi: 10.1136/annrheumdis-2016-210457: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5530353/
-
Singh et al. “Biologics or tofacitinib for people with rheumatoid arthritis unsuccessfully treated with biologics: a systematic review and network meta-analysis.” Cochrane Database Syst Rev. 2017 Mar 10;3:CD012591. doi: 10.1002/14651858.CD012591.: http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD012591/full
Look forward to seeing many of you in New York next week!
For those who will be in the Outcomes group at the F2F, please also take a look at this paper on MACE:
Looking forward to next week!!!
For those who will be helping with negative control selection next week I have have generated some documentation to help get you started:
This will be helpful for the MACE definition - outcome group
The Problem With Composite End Points in Cardiovascular …https://www.sciencedirect.com/science/article/pii/S0735109707036947
www.sciencedirect.com
Background. The term MACE is a commonly used end point for cardiovascular research. By definition, MACE is a composite of clinical events and usually includes end points reflecting safety and effectiveness.
Lisa Schilling, MD, MSPH
Professor of Medicine
Co-Director, Data Science to Patient Value Initiative
Medical Director, Office of Value Based Performance, CUMedicine
Department of Medicine, Division of General Internal Medicine
University of Colorado, School of Medicine
office AO1-Room 8219: 303-724-2254
office CUMed/UPI Building- ACCORDS: 303-724-
fax AO1: 303-724-2270
Mailing Address:
Division of General Internal Medicine
University of Colorado School of Medicine
8th Floor, Academic Office 1-Office 8219
Mailstop B180
12631 E. 17th Ave
Aurora, CO 80045
For those in the outcome definition group - here’s a first run at a MACE definition. It needs work but could be a starting point: http://www.ohdsi.org/web/atlas/#/cohortdefinition/1732315
My conclusions about MACE:
Little agreement among studies. In a string of about 10, none were identical.
Even looking at top journals, little agreement.
Have to separate cardiac and non-cardiac studies, where the cardiac ones get sophisticated about revacscularization, etc. We will use non-cardiac.
Have to separate RCT and retrospective. (RCT doesn’t use codes.)
No one, not even in top journals, validate MACE.
No one uses two codes for MACE or double checks inpatient status or troponin. But they may have specialized databases, such as inpatient only.
Seems like “3-point” MACE is good for us. It is defined as death (disagree on all cause or cardiovascular), MI, stroke (disagree on ischemic or hemorrhagic too).
Our definition was any of these:
-
MI with inpatient visit or with high troponin
-
Stroke ischemic and not hemorrhagic. We check that it is with inpatient visit or that it is with a head imaging study.
-
Death with a CV diagnosis.
Based on the above review, it would be reasonable to do all cause death, stroke without inpatient or imaging, or MI without inpatient or troponin. This would gather more patients and better match every other retrospective study of MACE I looked at. We are being more restrictive.
I don’t see anyone checking for two codes, especially because stroke and MI are episodes. For RA, I do see multiple codes used, but that’s a chronic disease.
And we could also validate, but that would be beyond the literature.