Team:
A little over a decade ago, a few colleagues got together to brainstorm an idea: there was excitement about the potential opportunities for expanding the use of observational data (administrative claims and electronic health records) to support medical product safety surveillance, but there was insufficient evidence to demonstrate that these data were reliable enough for the task, and it was clear that no one individual or organization from industry or academia or government had the breadth of skills and resources to conduct the methodological research necessary to establish scientific best practices for observational analyses. From those discussions came a public-private partnership chaired by the FDA, administered by FNIH, and supported by a consortium of pharmaceutical companies. The Observational Medical Outcomes Partnership (OMOP) was charged within designing and executing on a series of methodological experiments to answer specific questions all around a common theme: how much can we trust real-world evidence generated through the retrospective analysis of observational data? Our overall conclusion: if data were adequately standardized, if the appropriate statistical methods were applied, if study diagnostics passed and statistical estimates were empirically calibrated, if the findings compiled across disparate databases were generally consistent, then real-world evidence could be used to meaningfully inform medical decision-making.
About six years ago, a few colleagues got together to brainstorm an idea: there was excitement about the potential opportunities for expanding the use of observational data (administrative claims and electronic health records) to characterize disease natural history, estimate causal effects of medical interventions, and predict health outcomes, but it was clear that no one individual or organization had the breadth of skills and resources to establish an international data network, create an ecosystem of open-source analytics, and perform the analyses necessary to address the ever-expanding array of clinical and public health questions. From those discussions came a open science community, created by its collaborators, administrated by its collaborators, and supported by its collaborators. Observational Health Data Sciences and Informatics (OHDSI) wasn’t created because some researcher wanted to get a grant or write a paper, it wasn’t established to pacify the idle curiosities of informaticists and statisticians, it wasn’t formed to give industry a venue to obtain access to data for their commercial needs. OHDSI was created because several of us legitimately believed that we had the opportunity to improve health by empowering a community to collaboratively generate the evidence that promotes better health decisions and better care. That belief became OHDSI’s mission, and that mission is being put to the test right now.
According to the Johns Hopkins coronavirus resource center, as of 25March 8:44pmEST, there are 468,523 confirmed cases of COVID-19 and 21,192 deaths. We are facing a health crisis unlike anything most of us have ever seen in our lifetimes. We are in the midst of a pandemic that has torn through Asia and continues spread across Europe, North America, and South America, inflicting individuals in 173 different countries. Most of us now have friends and love ones directly impacted by the disease. Members of our own OHDSI community are currently ill with the virus, and our thoughts go out to them and wish them a healthy recovery.
One of the most difficult challenges of fighting this virus is that there is so much we don’t fully know about the COVID-19: what is happening to patients contract the virus? who is most at risk of complications? which treatments are most effective? And all of this uncertainty needs to be situated in the context of what we do know (or maybe don’t yet but could know) about our history with other viral diseases, symptoms, complications and associated impact to the healthcare system.
As a community, we have an opportunity - and an obligation - to do everything in our power to help fill these evidence gaps, reduce the uncertainty, and provide actionable information to inform policy makers, health care professionals, and patients. The OHDSI COVID-19 study-a-thon is an important milestone, because it represents the kickoff of a commitment we are making to each other to deliver on the promise of what’s possible when we collaboratively generate evidence to promote better health decisions and better care. We have a long journey ahead in our battle with COVID-19, but I hope you all take the next 4 days as part of the study-a-thon to recognize and appreciate that we are all on this journey together, and together we can make a major difference for public health.
All of you who registered for the OHDSI Study-a-thon should now have received an email invitation to join MSTeams. If you did register before the Monday midnight deadline and did not get an invitation, then please send an email to ohdsi-support@mi-erasmusmc.nl. If you did not register in time, we hope that you’ll follow along with our regular updates on OHDSI forums, Twitter, and LinkedIn.
Most of you who registered completed the ‘Collaborator focus area’ form. Those of you who self-identified preferences for specific ‘studies’ and ‘competencies’ will receive a separate email from me ‘assigning’ you to focus in those areas. Thank you for your input!
The official kick-off of the OHDSI COVID-19 Study-a-thon will take place on 26March2020 at 4pmKST/8amCET/7amGMT/3amET. Those registered should have received a calendar invitation to this Live Event, and those in OHDSI-COVID-19 MSTeams will also see the link to ‘Join’ under the General channel.
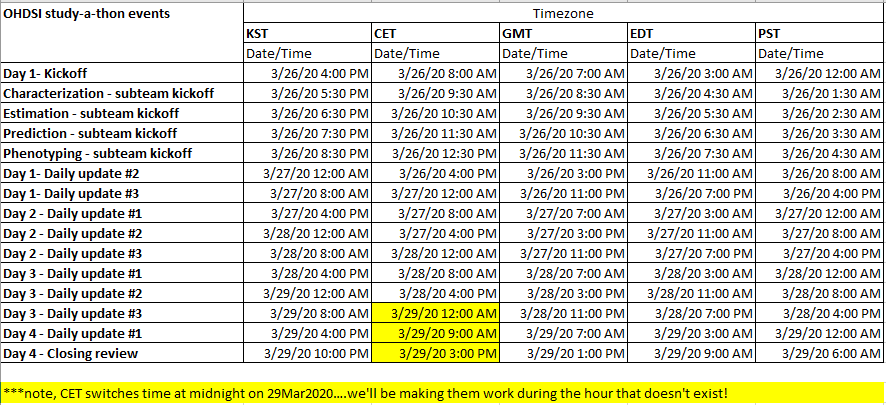
Here is the tentative calendar of scheduled meetings that will take place over the next four days. Similar to the kick-off, you’ll receive invitations for the ‘Daily Update’ meetings and can ‘join’ directly via MSTeams. All meetings are optional to attend, all are being recorded and will be posted after the session, so don’t feel like you’re missing out if you can’t make it. For the subteam kickoffs, I encourage everyone to only attend the one they are focused on, so that you can devote less time to ‘listening’ and more time to ‘doing’:
From here on out, all study-a-thon activities are to take place from within the MSTeams collaboration platform. This will be my last post on the OHDSI forums for a few days, as we turn our attention to generating evidence together. Thanks in advance to @CraigSachson for keeping those outside the study-a-thon apprised of our community activities by posting the recordings and writing up summary updates each day.
It’s an honor to have the chance to collaborate with all of you on this important problem at this important time. Let’s get to it.
Cheers,
Patrick