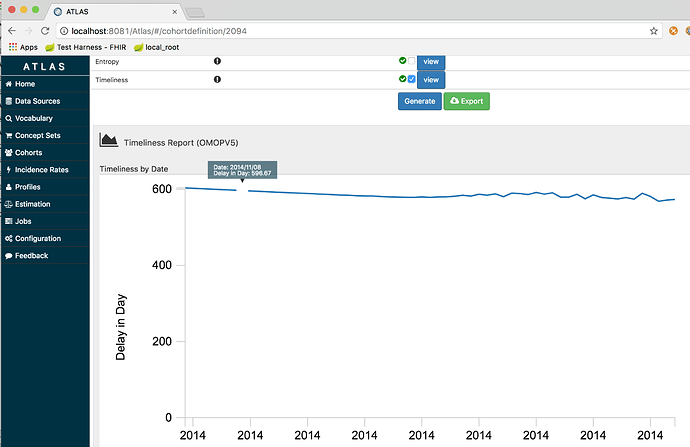
Greetings,
We have a public health researcher at Indiana University who is working to implement OHDSI / OMOP in the Public Health space. One of the areas that he is currently investigating is the measurement of timeliness that data is reported from a healthcare facility to a public health agency. In order to calculate timeliness, we need to be able to compare the observation time to the time the data was recorded (row created time). However, the created time is not currently part of the CDM. Could you please advise as to the proper workgroup or process to have the additional column added to the CDM? Please reference the following request from Dr. Dixon. Additionally, I would add that at the Regenstrief institute we have found the row_created_time and row_updated_time columns to be valuable to our incremental ETL strategy.
Timeliness is one of the most common dimensions of data quality studied in the information science literature [1]. Furthermore, the timeliness with which data are reported from a healthcare facility to a public health agency is a key aspect of measuring the effectiveness of a surveillance system [2,3]. Under a grant from the U.S. National Library of Medicine, we seek to extend the OHDSI framework to support measurement of data quality characteristics of importance to public health surveillance. To do so, we must make changes to the underlying database architecture to support the calculation of timeliness in OHDSI applications. Public health agencies would be strongly interested in routinely measuring the difference between the date that an observation was added to the database with the date of the observation. This will help the agencies measure how quickly data can be captured by the agency and loaded into the database for use by OHDSI and its surveillance systems. Many state laws require reporting by healthcare facilities within 24, 48, or 72 hours following diagnosis or an emergency room encounter. Measuring timeliness will allow health officials to monitor their ability to make these data available for surveillance following their transmission from healthcare facilities. Monitoring timeliness will help the agencies improve not only their internal processing of data but also improve the responsiveness from their healthcare facilities. Therefore we strongly urge your consideration of our request. We do not anticipate that the new fields will interfere or conflict with other fields already defined in the schema.
[1] Batini C, Cappiello C, Francalanci C, Maurino A. Methodologies for data quality assessment and improvement. Acm Comput Surv. 2009; 41(3).
[2] Buehler JW, Hopkins RS, Overhage JM, Sosin DM, Tong V. Framework for evaluating public health surveillance systems for early detection of outbreaks: recommendations from the CDC Working Group. MMWR Recomm Rep. 2004 May 7;53(RR-5):1-11.
[3] Dixon BE, McGowan JJ, Grannis SJ. Electronic laboratory data quality and the value of a health information exchange to support public health reporting processes. AMIA Annu Symp Proc. 2011; 2011:322-30.